
What are the isomers of cresols?
(A) ortho cresol
(B) meta cresol
(C) para cresol
(D) All of the above
Answer
570k+ views
Hint: Cresol is a compound known as Methyl phenol. It is obtained from coal tar or petroleum. Other names of Cresol are Hydroxytoluene. Depending on the temperature, it can be present as solid or liquid form. Cresol will have a low boiling point.
Complete Solution :
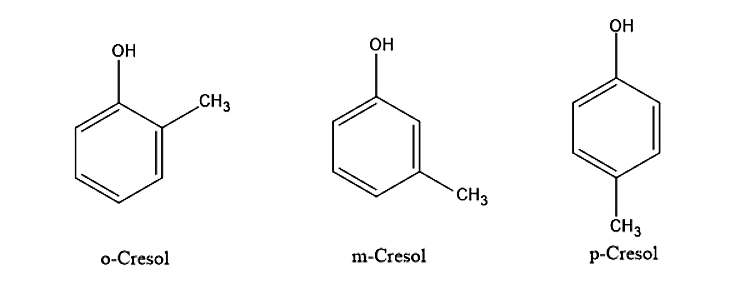
- Cresol is a chemical compound which is also known as the Methyl phenol. The other names of cresol are Hydroxytoluene. Based on the temperature cresol can be present in either liquid or solid form. This is because the melting point of the cresol is nearer to the room temperature. Cresol is obtained from petroleum or coal tar and therefore it will have a smell of coal tar. Cresol will be obtained as the mixture of three isomers. These isomers will have different structures. The isomers will be having methyl groups being substituted at different positions on the ring of phenol.
- When the methyl group is present at the ortho position of the phenol ring is called ortho cresol. Ortho cresols are also called as 2-Hydroxytoluene or 2- Methyl phenol. It is a colourless to white crystal. It can be found as a constituent in the smoke from tobacco.
- When the methyl group is at the meta position of the phenol ring it is called meta cresol. Meta cresols are also called as 3-Hydroxytoluene or 3- Methyl phenol. It is a colourless yellow liquid. m-Cresol is also found in the smoke of tobacco.
- When the methyl group is at the para position of the phenol ring it is called para cresol. Para cresols are also called 4-Hydroxytoluene or 4- Methyl phenol. It is a colourless prismatic crystal.
So, the correct answer is “Option D”.
Additional information:
Some of the application of o-Cresol, m-Cresol and p-Cresol are given below:
- o-Cresol can be used as a Calcium indicator, precursors to other compounds.
- m-Cresol can be used as a pesticide, solvent for polymers, preservatives for some insulins, antiseptics.
- p-Cresol can be used as an antioxidant due to their low-level toxicity.
Note: Exposure to cresol like applying, ingesting and inhaling can cause harmful effects such as
- It can irritate the nose.
- Damage the kidney, lungs, blood and liver.
- It can also cause burns in the skin.
- Nervous system damage.
- Vomiting.
Complete Solution :
- Cresol is a chemical compound which is also known as the Methyl phenol. The other names of cresol are Hydroxytoluene. Based on the temperature cresol can be present in either liquid or solid form. This is because the melting point of the cresol is nearer to the room temperature. Cresol is obtained from petroleum or coal tar and therefore it will have a smell of coal tar. Cresol will be obtained as the mixture of three isomers. These isomers will have different structures. The isomers will be having methyl groups being substituted at different positions on the ring of phenol.
- When the methyl group is present at the ortho position of the phenol ring is called ortho cresol. Ortho cresols are also called as 2-Hydroxytoluene or 2- Methyl phenol. It is a colourless to white crystal. It can be found as a constituent in the smoke from tobacco.
- When the methyl group is at the meta position of the phenol ring it is called meta cresol. Meta cresols are also called as 3-Hydroxytoluene or 3- Methyl phenol. It is a colourless yellow liquid. m-Cresol is also found in the smoke of tobacco.
- When the methyl group is at the para position of the phenol ring it is called para cresol. Para cresols are also called 4-Hydroxytoluene or 4- Methyl phenol. It is a colourless prismatic crystal.

So, the correct answer is “Option D”.
Additional information:
Some of the application of o-Cresol, m-Cresol and p-Cresol are given below:
- o-Cresol can be used as a Calcium indicator, precursors to other compounds.
- m-Cresol can be used as a pesticide, solvent for polymers, preservatives for some insulins, antiseptics.
- p-Cresol can be used as an antioxidant due to their low-level toxicity.
Note: Exposure to cresol like applying, ingesting and inhaling can cause harmful effects such as
- It can irritate the nose.
- Damage the kidney, lungs, blood and liver.
- It can also cause burns in the skin.
- Nervous system damage.
- Vomiting.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




